Abstract
Background: The Myelofibrosis and Essential Thrombocythemia Observational STudy (MOST) (NCT02953704) is an ongoing multicenter, noninterventional, longitudinal, prospective, observational study in patients with essential thrombocythemia (ET) or myelofibrosis (MF) enrolled in academic and community centers (ACs and CCs) throughout the United States. This analysis examined patient baseline characteristics, diagnosis, treatment patterns, and symptom burden in ACs vs CCs.
Methods: Eligible patients had high-risk (HR) (≥60 years of age and/or thromboembolic history) or low-risk (LR) ET (if receiving ET-directed therapy [not including aspirin only]); patients with MF (≥18 years of age) had LR MF or intermediate-1 (INT-1)-risk (INT-1R) MF by reason of age >65 years alone. Baseline data regarding demographics, diagnosis, treatment, and symptom burden were collected as previously characterized (Yacoub A. Clin Lymphoma Myeloma Leuk. 2021;21:461-69); this analysis compared data from 24 ACs with those from 82 CCs.
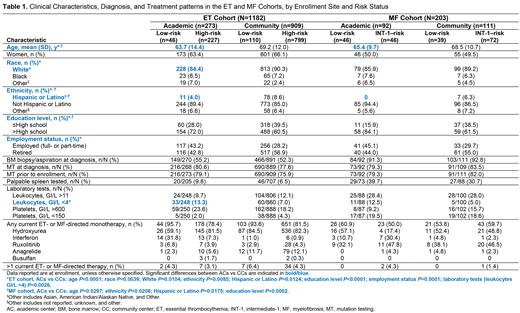
Results: In the ET cohort, 1182 of 1237 patients were evaluable for this analysis, with 273 AC (17% LR; 83% HR) and 909 CC (12% LR; 88% HR) enrollees. In the MF cohort, 203 of 232 patients were evaluable for analysis, with 92 AC (50% LR; 50% INT-1R) and 111 CC (35% LR; 65% INT-1R) enrollees.
Across both the ET and MF cohorts, the mean (SD) age was significantly higher in patients enrolled at CCs vs ACs (Table 1). In the ET cohort, significant differences were observed in patient race and ethnicity (both P<0.01) and significantly higher proportions of patients were White (P<0.05) and of Hispanic or Latino ethnicity (P<0.05) in CCs vs ACs. Significant differences were also observed in patient education level (P<0.0001) and employment status (P=0.0001) between ACs vs CCs, and a significantly higher proportion of patients were retired in CCs vs ACs (P=0.0001). In the MF cohort, a significant difference in ethnicity was observed (P<0.05) and a significantly higher proportion of patients were of Hispanic or Latino ethnicity in CCs vs ACs (P<0.05). Significant differences were also observed in patient education level in ACs vs CCs (P=0.0002), and a higher proportion of patients were retired in CCs vs ACs (P>0.05). At diagnosis, the proportion of patients with ET or MF reported to have undergone bone marrow evaluation and mutation testing for myeloproliferative neoplasms in ACs vs CCs was not significantly different at the 0.05 level. At enrollment, leukopenia was significantly more common in patients with ET enrolled at ACs vs CCs (P<0.01).
In the ET cohort, the proportion of HR patients who were receiving ET-directed monotherapy were similar between ACs and CCs (Table 1). Compared with ACs, higher proportions of LR and HR patients were receiving anagrelide, higher proportions of LR and HR patients were receiving hydroxyurea, and lower proportions of LR and HR patients were receiving interferon in the CCs. The proportion of patients with ≥1 ET-related physician-reported symptom was higher among ACs vs CCs (P=0.0001).
In the MF cohort, the proportion of LR patients receiving MF-directed monotherapy was higher in ACs vs CCs, while this proportion was lower in INT-1R patients in ACs vs CCs (Table 1). Compared with ACs, a higher proportion of LR patients were receiving anagrelide and ruxolitinib, a higher proportion of INT-1R patients were receiving hydroxyurea, and a lower proportion of LR and INT-1R patients were receiving interferon in CCs. The proportion of patients with ≥1 MF-related physician-reported symptoms was similar among ACs vs CCs (P>0.05).
Conclusion: These real-world data from MOST demonstrate that care was generally similar between ACs and CCs. Although there were key statistical differences in demographics (eg, age, ethnicity, and education level), disease features, and treatment history, the clinical significance of these differences remains unclear. Some of these differences (eg, demographics), however, could be attributed to the geographic location of the enrollee. Nevertheless, the older population and significant minority populations observed in CCs may represent a key population for clinical trial recruitment. Further investigation into these differences will help assess and enhance ET and MF disease management across sites.
Lessen: Astellas Pharma: Research Funding; Exact Sciences: Research Funding; Incyte Corporation: Consultancy, Research Funding, Speakers Bureau; Pfizer: Research Funding; Amgen: Speakers Bureau; Bayer: Consultancy, Speakers Bureau. Fazal: Sanofi Genzyme: Consultancy, Honoraria, Speakers Bureau; Novartis: Consultancy, Honoraria, Speakers Bureau; Karyopharm Pharmaceuticals: Consultancy, Honoraria, Speakers Bureau; Jazz Pharmaceuticals: Consultancy, Honoraria, Speakers Bureau; Janssen Oncology: Consultancy, Honoraria, Speakers Bureau; Taiho Pharmaceuticals: Consultancy, Honoraria, Speakers Bureau; Takeda: Consultancy, Honoraria, Speakers Bureau; Stemline Therapeutics: Consultancy, Honoraria, Speakers Bureau; Incyte: Consultancy, Honoraria, Speakers Bureau; Glaxo Smith Kline: Consultancy, Honoraria, Speakers Bureau; Gilead Sciences: Consultancy, Honoraria, Speakers Bureau; Bristol Myers Squibb: Consultancy, Honoraria, Speakers Bureau; AMGEN: Consultancy, Honoraria, Speakers Bureau; Agios: Consultancy, Honoraria, Speakers Bureau. Scherber: Incyte Corporation: Current Employment, Current holder of stock options in a privately-held company. Kalafut: Incyte: Current Employment, Current holder of stock options in a privately-held company. Ren: Incyte: Current Employment, Current holder of stock options in a privately-held company. Ritchie: NS Pharma: Research Funding; ARIAD Pharmaceuticals: Ended employment in the past 24 months, Speakers Bureau; Novartis: Consultancy, Honoraria, Other: travel support, Research Funding, Speakers Bureau; Takeda: Consultancy, Honoraria; Protaganist: Consultancy, Honoraria; Bristol Myers Squibb: Consultancy, Research Funding; Astellas: Consultancy, Research Funding; Celgene/BMS: Consultancy, Other: travel support, Speakers Bureau; Abbvie: Consultancy, Honoraria; Incyte: Consultancy, Honoraria, Speakers Bureau; Jazz: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal